The Ultimate Guide to Clinical Trial Management Systems
 Admin
Admin Software Development
Software Development Jan 08, 2025
Jan 08, 2025

Table of Content
Managing clinical trials has always been a challenging and difficult process. It covers a wide range of duties, including patient enrollment, financial monitoring, and regulatory compliance. In the old days, most of these jobs were completed with spreadsheets, although they usually took a long time.
With the emerging technology, clinical trial management solutions have changed a lot. Be it a large network of research sites or a single clinical site, CTMS solutions have become the new cornerstone to streamline operations, enhance compliance, and increase efficiency in clinical research.
What is a CTMS - Clinical Trial Management System?
A Clinical Trial Management System (CTMS) is a custom software platform designed to streamline the management of clinical trials. It works as a hub for organizing, tracking, and overseeing all aspects of a trial. From patient enrollment and scheduling to regulatory compliance and financial management, CTMS does it all.
By replacing old ways like spreadsheets, a CTMS increases efficiency and accuracy among various research teams through enhanced collaboration. It offers end-to-end insights in real-time, which would make it necessary for a clinical research site of any size.
Who Needs a CTMS?
Here's a closer look at who benefits from a CTMS
1. Clinical Research Organizations (CROs)
To handle several facets of clinical trials, such as participant recruitment, data collecting, regulatory compliance, and project timeframes, CROs depend on CTMS platforms. These tools assist CROs in improving trial results, streamlining procedures, and lessening administrative load.
2. Businesses in Biotechnology and Pharmaceuticals
For improved team coordination, resource optimization, and adherence to the strictest regulatory requirements, these firms use CTMS systems.
CTMS ensures that the trial is completed on time with live progress tracking, financial monitoring, and central document storage to support efficient operation.
3. Academic Research Institutions
The use of CTMS by the universities and the academic centers benefits in organizing and managing complex processes in conducting clinical research. Improved data accuracy, better participant management, and compliance with regulatory requirements can be achieved in this way.
4. Health Systems and Site Networks
Hospitals, health systems, and site networks conducting clinical research find CTMS invaluable for managing trial sites, maintaining compliance, and streamlining operations. Integration with existing systems, such as electronic medical records (EMRs), enhances billing compliance, improves patient safety monitoring, and saves significant time.
5. Independent Research Sites
Small or independent research sites use CTMS to increase efficiency in the recruitment, tracking, and communication of patients. The tools assist in improving patient engagement, reducing no-shows, and optimizing recruitment strategies to maximize the success of the trial.
Benefits of a CTMS
- Operational Efficiency A CTMS automates the task management of the trials: reduces administrative workloads; improves coordination at multiple sites: ensures quick task completion; and centralizes access to information.
- Data Accuracy and Availability The use of a CTMS minimizes manual errors, which ensures accurate and reliable data. It provides a centralized data repository for better decision-making and stronger trial outcomes.
- Regulatory Compliance CTMS ensures that legal and regulatory compliance is maintained through secure data handling, automated alerts, and detailed reports, which ensure transparency and protocol compliance.
- Monitoring and Transparency Real-time tracking allows early issue detection, prevents participant duplication, and enhances communication among stakeholders for smoother trial management.
- Centralized Data Management With secure, centralized data storage, a CTMS simplifies data access, eliminates redundancy, and streamlines workflows for efficient reporting and collaboration.
Main Features of a CTMS
A clinical trial management software streamlines complex clinical trial management processes with diverse features. The complexity may differ from one trial to another, but most core capabilities of the CTMS include:
1. Data Collection and Input
- Collects data from participants and input by staff or research associates.
- Centralizes data for easy access and organization.
2. Data Management and Analysis
- Manages data across multiple studies using tools provided.
- Automates data analysis for actionable insights.
3. Reporting Tools
- Generates comprehensive reports from the trial data.
- Produces reports to sponsors, and regulatory bodies such as the FDA, independent review boards, and IRBs.
4. Regulatory Support
- Supports compliance through data preparation and export to regulatory systems like the FDA's database.
- Aids in the monitoring and reporting of safety data.
5. Data Archiving and Retrieval
- Archives historical data safely for later access.
- Streamlines the retrieval process for archived data when conducting audits or reviews.
6. Integration with External Systems
- Exports trial data to external systems for regulatory and sponsor requirements.
How To Develop A Clinical Trial Management System?
Here is a step-by-step guide to developing a CTMS:
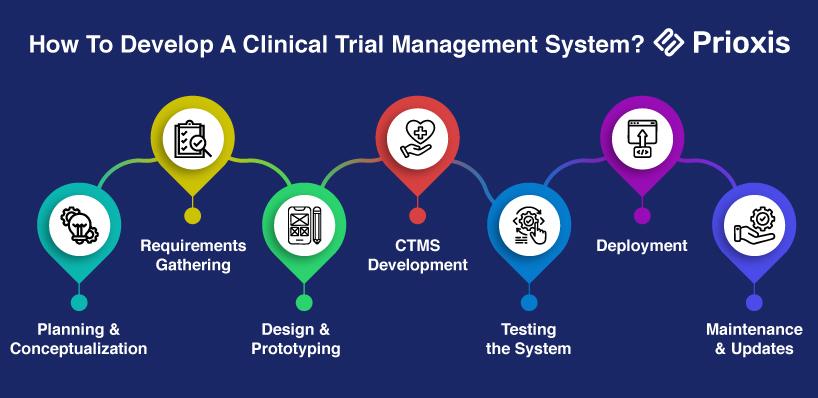
1. Planning and Conceptualization
Start by outlining the vision and purpose of the CTMS. Partner with clinical research professionals to recognize the industry's pain points and to find ways of solving them. Key considerations during this phase are:
- Identify researcher pain points and possible solutions.
- Determine data collection and analysis techniques.
- Understand regulatory and compliance expectations.
- Define the budget and determine the expertise required.
2. Requirements Gathering
Engage with stakeholders such as sponsors, researchers, and regulatory bodies to gather insights through workshops, surveys, and interviews. This helps define the system's scope and ensures all gaps and expectations are addressed. Document a roadmap for the development process, ensuring alignment with user needs.
3. Design and Prototyping
Design and intuitively prototype the CTMS to improve user experience. Collect feedback from stakeholders and refine the design to fit user preferences. This stage involves developing a user-friendly interface and efficient workflows.
4. CTMS Development
Develop the CTMS by:
- Frontend Development: Developing a responsive and user-friendly interface.
- Backend Development: Developing system logic, databases, and server infrastructure.
- System Integration: Seamless interface with external systems, such as clinical trial registries, EDC, and regulatory databases
5. Testing the System
System testing validation: The test ensures that CTMS functions appropriately, is secure, and meets regulatory requirements. Testing phases:
- Unit testing
- Integration testing
- System testing
- Regulatory compliance testing
- User acceptance testing
6. Deployment
Prepare the live environment, transfer data, and implement the CTMS in a real-world setting. Monitor the system during launch to resolve any issues and ensure smooth operation. Train users thoroughly on how to utilize the system effectively.
7. Maintenance and Updates
Ensure the CTMS remains functional and up-to-date by:
- Monitoring for performance issues regularly.
- Updating the system to comply with evolving regulations.
- Adding new features based on user feedback.
- Providing continuous support to address user concerns.
Conclusion
A clinical trial management system is a crucial instrument in the conflict for effectiveness, accuracy, and creativity in the field of clinical trials. Among many other advantages, this reduces risks and errors, automates a lot of jobs, simplifies operations, and conforms with regulations.
By integrating essential systems and leveraging advanced technologies, stakeholders can manage medical processes more professionally and expeditiously.
As you consider building a clinical research management system to make life easier and enable streamlined operations, keep the bigger vision in mind. What are the specific pain points you are looking to address through this software? what is clinical trial management and the future direction of such a system?
While the development process may seem complex, working with a company like Prioxis ensures that you will get an agile, flexible, and scalable CTMS fully functional for your needs. At Prioxis, let us partner to simplify the clinic trials so that you can be successful in your research.
Get in touch
